- Fensolvi is the only six-month, subcutaneously administered leuprolide acetate approved for the treatment of pediatric patients two years of age and older with Central Precocious Puberty
- FDA approval was based on data from a multicenter, open-label, single-arm Phase 3 study with twice-yearly dosing
- Fensolvi demonstrated clinical efficacy in suppressing sex hormone levels and arresting or reversing progression of puberty with a favorable safety and tolerability profile
BUFFALO GROVE, Ill., May 4, 2020 /PRNewswire/ -- Tolmar Pharmaceuticals, Inc. announced today that the U.S. Food and Drug Administration (FDA) has approved its New Drug Application for FENSOLVI® (leuprolide acetate) for injectable suspension for the treatment of pediatric patients two years of age and older with central precocious puberty (CPP). CPP is a rare disease defined as the onset of puberty before age eight in girls and before age nine in boys.
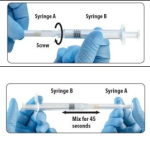
Leuprolide acetate is the most widely used treatment for CPP. Fensolvi utilizes an innovative proprietary polymeric gel technology that forms an in-situ solid after injection and releases leuprolide acetate in a sustained and controlled manner over time. This polymeric gel technology enables a small volume of injection of only 0.375mL, subcutaneous administration, and a six-month dosing cycle.
"We are very enthusiastic about our clinical data demonstrating the efficacy and safety of Fensolvi. The six-months dosing schedule, small volume of injection, and subcutaneous administration are important new product features," said Dr. Stuart Atkinson, MB ChB, Vice President and Head of Medical Affairs, Tolmar Pharmaceuticals.
Fensolvi Efficacy and Safety
FDA approval was based on results from a multicenter, open-label, single arm Phase 3 study evaluating the efficacy, safety and pharmacokinetics of leuprolide acetate (LA) 45 mg for injectable suspension in 64 children with central (gonadotropin-dependent) precocious puberty. The study achieved its primary endpoint, with 87 percent of children achieving a serum luteinizing hormone concentration of <4 IU/L at six months post injection. The study also demonstrated that Fensolvi suppressed sex hormones to pre-pubertal levels, and stopped or reversed the progression of clinical signs of puberty.
"Children with CPP require treatment for several years and missing treatment or stopping treatment too soon may lead to significant short stature and misalignment between chronological age and physical and emotional development," said Karen Klein, M.D., Associate Clinical Professor, Rady Children's Hospital, University of California San Diego. "Fensolvi offers treating physicians and their patients with CPP a safe and effective treatment option that is administered twice a year with a small injection volume that has the potential to improve compliance."
Treatment emergent adverse events (TEAEs) were mostly mild or moderate, with none leading to withdrawal from the study. The most common TEAEs were injection site pain (31%), nasopharyngitis (22%), and fever (17%).
About Central Precocious Puberty (CPP)
Gonadotropin releasing hormone (GnRH)-dependent central precocious puberty (CPP) is the premature development of signs of sexual maturation occurring in girls younger than eight and in boys younger than nine years of age. CPP patients are at risk of having significantly short stature as adults in addition to social, psychological and emotional issues, including lower self-esteem, stress, anxiety and depression, all of which may negatively impact quality of life. CPP is believed to have a five- to twenty-fold higher incidence in girls than in boys and is estimated to occur in one in 5,000-10,000 children.
About Fensolvi® (leuprolide acetate) for injectable suspension
Fensolvi (leuprolide acetate) for injectable suspension is a gonadotropin releasing hormone (GnRH) agonist indicated for the treatment of pediatric patients two years of age and older with CPP. It is a prescription drug, given by injection.
Fensolvi is the first and only six-month, subcutaneous leuprolide acetate with a small injection volume that allows flexibility in selection of injection site and enables administration in an office setting. Fensolvi represents an effective, safe and convenient treatment option for this vulnerable patient group, and its profile aligns with twice-yearly visits to the pediatric endocrinologist's office.
Information about FENSOLVI is available at fensolvi.com.
Important Safety Information for Fensolvi® (leuprolide acetate) for injectable suspension
FENSOLVI (leuprolide acetate) for injectable suspension is a gonadotropin releasing hormone (GnRH) agonist used to treat patients 2 years of age and older with central precocious puberty (CPP). CPP may be diagnosed when signs of sexual maturity begin to develop in girls under the age of 8 or in boys under the age of 9.
FENSOLVI is contraindicated in individuals with hypersensitivity to any drug that is in the same class as FENSOLVI, in individuals who are allergic to any of the ingredients in FENSOLVI, or in individuals who are pregnant. FENSOLVI may cause fetal harm when administered to a pregnant patient.
During the first few weeks of treatment, increases in gonadotropins and sex steroids above baseline may result in an increase in signs and symptoms of puberty including vaginal bleeding in girls.
Psychiatric events have been reported in patients taking GnRH agonists. Events include emotional lability, such as crying, irritability, impatience, anger, and aggression. Patients should be monitored for development or worsening of psychiatric symptoms.
Convulsions have been observed in patients treated with GnRH agonists with or without a history of seizures, epilepsy, cerebrovascular disorders, central nervous system anomalies or tumors, and in patients on concomitant medications that have been associated with convulsions such as bupropion and SSRIs.
The most common adverse events seen with FENSOLVI were: injection site pain, nasopharyngitis, pyrexia, headache, cough, abdominal pain, injection site erythema, nausea, constipation, vomiting, upper respiratory tract infection, bronchospasm, productive cough and hot flush.
Please see Full Prescribing Information for FENSOLVI for additional important safety information.
To report suspected adverse reactions contact Tolmar at 1-844-4TOLMAR (486-5627) or the FDA at 1-800-FDA-1088 or visit www.fda.gov/medwatch.
About Tolmar
Tolmar is a fully integrated pharmaceutical company focused on the innovative development, approval, manufacturing and commercialization of specialty pharmaceuticals.
"Tolmar" refers to Tolmar Holding, Inc. and its wholly owned operating subsidiaries, Tolmar Inc., Tolmar Therapeutics, Inc., and Tolmar Pharmaceuticals, Inc. Tolmar global headquarters, product development and manufacturing facilities are based in northern Colorado, while Tolmar Pharmaceuticals' U.S. commercial business is based in Buffalo Grove, Illinois. For more information about the company, please visit www.tolmar.com.